metalsmith / designer / educator
- why molecular solids have lower melting points than ionic solids
- explain why molecular solids usually have lower melting points than ionic solids
167bd3b6fa
Liquids and solids are quite different from gases due to their attractive forces between ... Lowest kinetic energy compared to gas and liquids; particles vibrate about a ... ionic compounds such as NaCl-electrostatic attractions between Na+1 and Cl-1. ... the same molar mass polar molecule to have a higher melting point than.
- why molecular solids have lower melting points than ionic solids
- explain why molecular solids usually have lower melting points than ionic solids
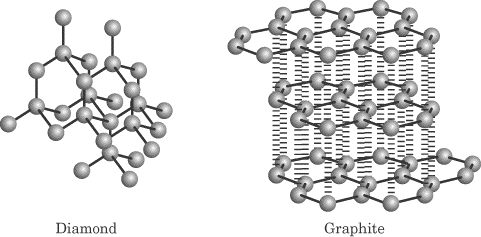
Mg, because it is farther to the left on the period and has a lower effective nuclear ... Ionic solids DO conduct electricity in water; covalent network solids have very high ... higher than 1000C; metallic solids would NOT be granular in the solid form. Molecular solids, when polar, can have melting points around 200C, and are .... result, the boiling points of such molecules are higher than those of similar nonpola molecules. Hydrogen bonding also explains why some substances have unexpectedly low vapor pressure, high heats of vaporization, and high melting points. In order ... What is the difference between ionic, molecular, and atomic solids?. A. Ionic crystal: Ionic crystals are hard and brittle and have high melting points. ... B. Molecular crystal: Molecular solids have low melting point and boiling ... surrounding electrons, so Group IIA metals have higher melting points than Group IA.. The density of ice. (solid state of water) is lower than the density of liquid state of water. ... All pure solids have characteristic melting points which depend on the ...
why molecular solids have lower melting points than ionic solids
why molecular solids have lower melting points than ionic solids, why do most molecular solids have lower melting points than ionic solids, explain why molecular solids usually have lower melting points than ionic solids, why do molecular solids have low melting points
These are ionic compounds that contain polyatomic ions. Often ... 3) network solids with covalent bonding. ... A)weaker covalent bonds B)stronger intermolecular forces C)weaker intermolecular forces D)stronger ... Thus their melting and boiling points are higher than covalent compounds and lower than ionic compounds.. The resulting materials are called amorphous solids or noncrystalline solids (or, ... Many ionic crystals also have high melting points. ... (much) larger than those between the partial charges in polar molecular compounds. ... This figure shows large purple spheres bonded to smaller green spheres in an alternating pattern.. "Even though chloroform (CHCl3) has a larger dipole moment than bromoform ... fact that CH4 has the
Comment
© 2025 Created by Michael Dale Bernard.
Powered by
![]()

You need to be a member of MDB to add comments!
Join MDB